Salonpas® Pain Relief Patch LARGE
The First FDA-Approved OTC Topical Pain Reliever
- Powerful Relief that lasts up to 12 hours
- Clinically proven for mild to MODERATE pain relief
Number of Patches: 9 Count
Patch Size: 3.94" × 5.50" (10cm x 14cm)
Active Ingredients (Per Patch)
Menthol 3% (Topical analgesic)
Methyl salicylate 10% (NSAID*) (Topical analgesic)
* nonsteroidal anti-inflammatory drug
Drug Facts
- Uses
- Temporarily relieves mild to moderate aches & pains of muscles & joints associated with:
- Strains
- Sprains
- Simple backache
- Arthritis
- Bruises
- Warnings
- For external use only
- Stomach bleeding warning:
- This product contains an NSAID, which may cause stomach bleeding. The chance is small but higher if you:
- Are age 60 or older.
- Have had stomach ulcers or bleeding problems.
- Take a blood thinning (anticoagulant) or steroid drug.
- Take other drugs containing an NSAID (aspirin, ibuprofen, naproxen, or others).
- Have 3 or more alcoholic drinks every day while using this product.
- Take more or for a longer time than directed.
- Do not use:
- On the face or rashes.
- On wounds or damaged skin.
- If allergic to aspirin or other NSAIDs.
- With a heating pad.
- When sweating (such as from exercise or heat).
- Any patch from a pouch that has been open for 14 or more days.
- Right before or after heart surgery.
- Ask a doctor before use if:
- You are allergic to topical products.
- The stomach bleeding warning applies to you.
- You have high blood pressure, heart disease, or kidney disease.
- You are taking a diuretic.
- When using this product:
- Wash hands after applying or removing patch. Avoid contact with eyes. If eye contact occurs, rinse thoroughly with water.
- The risk of heart attack or stroke may increase if you use more than directed or for longer than directed
- Stop use and ask a doctor if:
- You feel faint, vomit blood, or have bloody or black stools. These are signs of stomach bleeding.
- Rash, itching or skin irritation develops:
- Condition worsens.
- Symptoms last for more than 3 days.
- Symptoms clear up and occur again within a few days.
- Stomach pain or upset gets worse or lasts.
- If pregnant or breast-feeding:
- Ask a health professional before use. It is especially important not to use methyl salicylate at 20 weeks or later in pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
- Keep out of reach of children
- If put in mouth, get medical help or contact a Poison Control Center right away. Package not child resistant. Dispose of the used patches after folding sticky ends together.
- Directions
- Adults 18 years and older:
- Clean and dry affected area.
- Remove patch from backing film and apply to skin (see illustration).
- Apply one patch to the affected area and leave in place for up to 8 to 12 hours.
- If pain lasts after using the first patch, a second patch may be applied for up to another 8 to 12 hours.
- Only use one patch at a time.
- Do not use more than 2 patches per day.
- Do not use for more than 3 days in a row.
- The used patch should be removed from the skin when a new one is applied.
- Children under 18 years of age:
- Do not use; this product has not been shown to work in children
- Other Information
- Some individuals may not experience pain relief until several hours after applying the patch.
- Avoid storing product in direct sunlight.
- Protect product from excessive moisture.
- Store at 20-25°C (68-77°F).
- Inactive Ingredients
- Alicyclic Saturated Hydrocarbon Resin, Backing Cloth, Film, Mineral Oil, Polyisobutylene, Polyisobutylene 1,200,000, Styrene-Isoprene-Styrene Block Copolymer, Synthetic Aluminum Silicate
Clinical Studies
Higashi Study Highlights & Related Information
A randomized, double-blind, parallel-group, placebo-controlled, multicenter study, reviewed and confirmed by the FDA’s New Drug Application process, means this is an OTC topical analgesic that has the clinical proof and approval few have.
Strong Clinical Trial Results
40% Additional relief experienced with Salonpas® vs Placebo
58% Study patients rating satisfaction from Salonpas® as good/very good/excellent
From a randomized, double-blind, parallel-group, placebo-controlled, multicenter study1
Passed Rigorous FDA Review
<10% Less than 10% of OTC drug products have an NDA or have even been reviewed by the FDA
Salonpas® is the first OTC topical pain treatment to earn an NDA
The FDA’s New Drug Application (NDA) process is a rigorous process that every prescription medicine since 1938 has passed through.
Salonpas® is the first OTC labeled to treat both mild and moderate pain
Near-Zero Side Effects
.001% Rate of adverse events to Patch sales2
Long-Lasting, Controlled Dose
Treatment works locally, significant pain relief can start within 1 hour and lasts for up to 12 hours, with very little medicine traveling throughout the bloodstream. Some individuals may not experience pain relief until several hours after applying the patch.
For a published study reprint, please contact us using the button below.
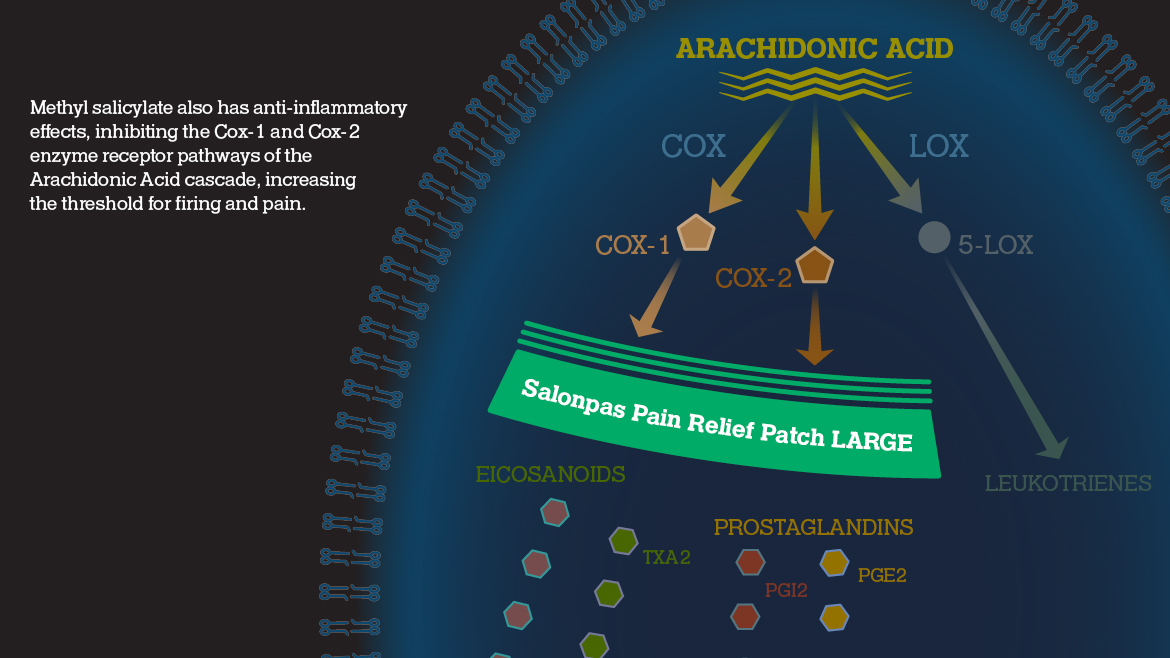
Mechanism of Action
Salonpas® Pain Relief Patch LARGE interrupts key pathways
Salonpas® Pain Relief Patch LARGE is believed to have multiple mechanisms of action with counterirritant effects contributed by both methyl salicylate and menthol.